The Study on the Corrosion of 22MnB5 Hot-Forming Parts
Pressler independent research and owns the proprietary intellectual property rights: CN109821951A, CN109136907A, CN109021630A
Abstract:
The different manufacturing processes of 22MnB5 hot-forming parts were studied, including hot-forming tests of non-coated sheet, Al-Si coated sheet , GA sheet and electro galvanized parts of non-coated sheet after hot-forming.
Coating morphology was studied by means of optical microscope. Neutral salt spray experiments for 720h were carried out on non-coated sheet, Al-Si coated sheet, GA sheet and electro galvanized parts of non-coated sheet after hot-forming before and after electrophoresis.
The results showed that the electrophoretic (E-coating) layer had good corrosion resistance, but not for round holes, cutting edges. The weight test for 720h neutral salt spray before E-coating showed that the corrosion resistance of electro galvanized parts of non-coated sheet after hot-forming was 20 times stronger than that of GA and Al-Si sheets (calculated by area weight loss).The scratch test showed that the corrosion resistance of non-coated sheet by hot-forming was the worst and corroded along the thickness direction after E-coating, the Al-Si coated sheet by hot-forming corroded along the thickness and transverse directions because of the lower electrode potential than non-coated sheet. GA sheet by hot-forming and electro galvanized parts of non-coated sheet after hot-forming were corroded along the transverse direction because of sacrificing anode effect, and the substrate was not corroded.
0 Introductions
When the E-coating of the car body is damaged, the exposed metal parts are easy to corrode and rust. In addition, in the case of environmental pollution areas or bad weather conditions, such as spraying salt on roads in winter, it will speed up the corrosion of the car body. Or when the E-coating cannot reach the areas of the body structure, such as the overlap area of spot welding, it will cause internal corrosion of the body. These problems have not been resolved well when the corrosion is caused inside the car body.
Hot-forming steel (PHS) has the advantages of reducing body weight and improving crash safety. It is widely used in the auto body structures. However, decarburization and oxidation peeling are easy to occur on the surface of the sheet during heating. To solve this problem, researchers have developed different hot-forming coatings [1-4]. At present, the earliest developed and commercialized hot-formed steel with coatings is the Al-Si coatings produced by Arcelor Company in Europe. The Al-Si coating has a good high temperature resistance and corrosion resistance, but the coating layer is easy to be damaged and the base material is exposed when heated and formed at high temperature. Moreover, the coating does not provide anti-corrosion on cutting edges.
In recent years, major automobile manufacturers have shifted their research and development focus to zinc-based coatings with unique sacrificial anode and cathodic protection. Zinc-based coatings have low cost, excellent anti-corrosion performance and adequate oxidation resistance. They are the ideal anti-corrosion coatings for automobile body parts. In hot forming applications, however, GI or GA has not seen widely used in large scale of auto parts as it is seen in the cold forming parts, except galvanized sheets are in small scale used in indirect hot forming process. In the process of hot forming, the melting point of zinc-based coating is lower than steel substrate. When formed at high temperature, liquid zinc will easily lead to liquid metal brittlement, causing cracks in the base parts. In many researches people tried zinc coating in the direct hot forming process in which the heated blank is formed at the lower temperature compared with Al-Si coating, however, the mechanical properties of parts will be reduced [5-6].
At present, research on hot-forming coating mainly focuses on the oxidation resistance of the coating, but there are few reports about the corrosion resistance of the coating. In this work, the corrosion properties of Al-Si and GA sheets by heating, hot-forming and quenching, and electrogalvanized parts of non-coated sheet after hot-forming and quenching were studied. Their corrosion properties were compared.
1 Test materials
The test materials are 22MnB5 of GA sheet, Al-Si coated sheet, non-coated sheet and electro galvanized parts of non-coated sheet after hot-forming. The test equipments are flat sheet die, 315T hot-forming machine, salt fog test box, metallographic microscope and so on.
Table 1: 22MnB5compositions
|
Steel Grade |
C |
Si |
Mn |
Cr |
Ti |
B |
Ni |
|
22MnB5 |
0.22 |
0.2 |
1.25 |
0.2 |
0.03 |
0.003 |
0.003 |
Table 2: Coating type and thickness
|
Coating |
Uncoated |
Al-Si |
GA |
Electrogalvanized parts of non-coated sheet after hot-forming |
|
Name |
--- |
AS 75/75 |
GA 70/70 |
--- |
|
thickness |
0 |
30um |
10um |
10um |
2 Test Conditions and Processes
2.1 Samples Preparation
First, Al-Si coated sheet, GA steel and non-coated sheets were cut into small pieces of 80mm*200mm by laser cutting. The sample of sheets were heated for 240 seconds in a resistance heating furnace at 930℃, through completely austenitized and then air-cooled or quenched by 315T hot-forming machine. The GA steel is easy to volatilize at high temperature. Therefore, the GA steels were putted into the resistance heating furnace at 880℃, heated for 240 seconds and then hot-forming at 550℃. The experimental conditions were shown in Table 3.
2.2 Metallographic observation of coating morphology
The samples were reduced into suitable size and then sealed. After solidification, the metallographic specimens were corroded by 4% nitric acid and ethanol, and the corrosion time was about 10-12 seconds. The coating morphology of the raw materials and coating morphology after hot-forming were observed by metallographic microscope.
2.3 Neutral Salt Spray Experiment
2.3.1 Loss experiment of hot-forming sheets for 720h salt spray weight
The22MnB5 non-coated sheet, Al-Si coated sheet, GA sheet by hot-forming and electrolytic galvanized parts of non-coated sheet after hot-forming were subjected to 720h salt spray test, and the weight was measured before the experiment. After the experiment, they were measured again after removing the rust.
2.3.2 Scratch diffusion experiment of E-coating
The22MnB5 non-coated sheet, the Al-Si coated sheet, the GA sheet of hot-forming, and electro galvanized parts of non-coated sheet after hot-forming were subjected to E-coating treatment, and then scratched to two 150mm long crossover lines by a 1.0mm wide blade. The scratch cuts through the coating to expose the substrate. Theoriginal scratch width is measured. The scratch width was measured againafter720h neutral salt spray experiment.
2.3.3 B-pillars with different coatings
B-pillars of non-coated and Al-Si sheets were hot-formed and non-coated B-pillars were galvanized. Neutral salt sprayed experiments on different B-pillars were carried out.
3 Test results and analysis
3.1 Metallographic Analysis
Figures 1 to 7 showed the coatings morphology with magnification of 500 times. Table 3was the coating thickness dates of the experimental samples (Figures 1 to 7).
Fig. 1 shows the coatings morphology of the raw material of Al-Si sheet. The coating thickness was about 30um. It mainly consisted of Al-Si layer and transition layer Al-Fe-Si. The thickness of Al-Fe-Si layer was 7um, and the coating structure was dense and smooth. After 240 seconds of heating at 930℃, the Al-Si coating diffused in the substrate, and the thickness of the coating grows to 40 um. After heating, the surface coating was cracked and there were small holes in the coating, as showed in figure2. After hot-forming, the thickness of Al-Si coating and transition layer remained unchanged, but the quality of the coating was seriously reduced, as showed in figure3. Li Bing et al. [7] also confirmed that after hot-forming, the coating changed from a dense metal compound structure to a six-layer metal compound structure. A large number of cracks are produced in the coating. The more intense the coating fell off, and the more scratches on the surface of the parts, the easier to cause parts to break. And adhesion friction between the coating and the die occurred. Guizhongxiang et al. [8] also studied the hot-forming formability of boron steel sheet with Al-Si coating by tensile test, which showed that after hot stretching deformation, a large number of cracks appeared on the surface coating of boron steel.
Fig. 4 shows the metallographic morphology of the raw material of GA sheet. The coating thickness was 10um. It was mainly composed of pure zinc layer and zinc-iron alloy layer. The structure of the coating was compact and smooth. After 240 seconds of heating at 880℃ in the furnace, the galvanized layer diffused with the iron element in the steel base, and the thickness of the coating was increased to 19 um, but the galvanized layer was ruptured seriously. After hot-forming, the surface of the galvanized layer was uneven and its thickness was between 9um and 20um, because of a large amount of galvanized coating was adhered to the hot-forming die during hot-forming, as showed in figs. 8 and 9. Qiu Xiaopan, Zhang Jie and others also [9] founded that the thickness of GI coating increased significantly after heating, and the interface between coating and steel substrate was blurred. The structures of the coatings were ζ phase and δ phase after heating at 500℃. With the increase in heating temperature, the structure of the coating was transformed into Г phase with more iron content. Most of the coatings was transformed into Fe-Zn alloy phase when the temperature was above 880℃. The micro-cracks also occurred in the steel substrate under the tip stress of the cracks at the friable Fe-Zn alloy phase.
Fig.7 shows the cross section of the coating for electrogalvanized parts of non-coated sheet after hot-forming. It could be seen from the figure that the electroplated zinc layer was dense and uniform, and without pores, the same as the original material of the GA sheet, and the coating quality was much better than that of the Al-Si sheet and the GA sheet by hot-forming.
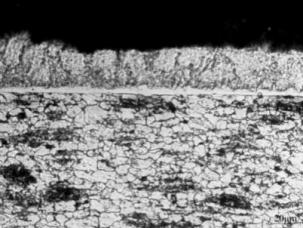
Figure 1
|
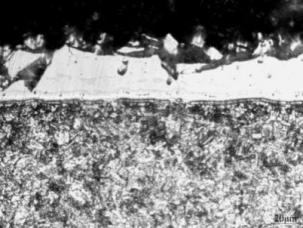
Figure 2
|
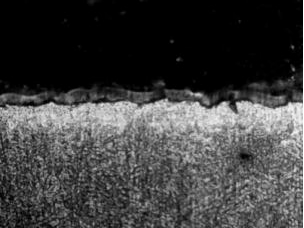
Figure 3
|

Figure 4
|
 Figure 5 |
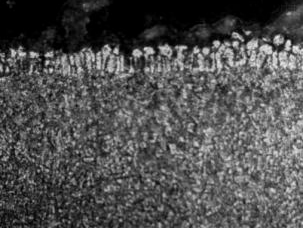 Figure 6 |
 Figure 7 |
Figure 1: raw material of Al-Si steel
Figure:2:heating and air cooling of Al-Si steel
Figure 3: hot-forming of Al-Si steel
Figure 4: raw material of GA steel
Figure 5:heating and air cooling of GA steel
Figure 6: hot-forming of GA steel
Figure 7: electroplated zinc parts of non-coated sheet after hot stamping
Table 3: Coating thickness of test samples
|
sample |
raw material of Al-Si steel |
heating and air cooling of Al-Si steel |
hot-forming of Al-Si steel |
raw material of GA steel |
heating and air cooling of GA steel |
hot-forming of GA stee |
electro galvanized sheet after hot-forming |
|
thickness |
30um |
40um |
7um-40um |
10um |
19um |
9um-20um |
9um-13um |
3.2 Salt spray experiment of Coating
3.2.1 Weight loss experiment after hot-forming for 720h salt spray test
It could be seen from Table 4 and Figure 8 to 11, the corrosion resistance of non-coated sheet by hot-forming was the worst(weight loss per unit area was 1.9E-3g/mm2), followed by GA sheet, Al-Si sheet was slightly better, and the best was the electrogalvanized parts of non-coated sheet after hot-forming. The corrosion resistance of electrogalvanized parts of non-coated sheet after hot-forming was 20 times higher than that of Al-Si and GA sheet (weight loss per unit area).
Mali et al. [10] carried out electrochemical tests of NaCl solution. It was noted that the Al-Si coating was composed of multi-layer metal compounds after heating. The first layer of Fe2Al5 began to corrode at the earliest time, and the electrochemical potential for other coatings was basically the same as that of the substrate. This stated that the Al-Si coating could not provide cathodic protection, so the areas without coating such as trimming were easy to corrode. The corrosion rate of Al-Si coating by hot-forming was slower than that of steel substrates. It was caused by the filling of corrosion products of Fe-Si-Al in corrosion holes, which hindered the diffusion and corrosion of oxygen.
The results of M. Fleschanderl et al. [11] showed that the electrochemical potential for the Fe-Zn intermetallic compound layer in the superficial layer was about -0.75V and the Fe-Zn solid solution layer in the bottom was about -0.6V, higher than that of the common GI and GA coatings, but still much lower than that of the steel substrate (-0.42V). Coatings after hot-forming still had good cathodic protection and corrosion resistance.
And people also believe that the corrosion resistance of the GA sheet is less than that of the GI sheet because of the more iron content in GA coating compounds.
Since the surface of electro galvanized parts of non-coated sheet after hot-forming was dense and smooth, there were no problems such the surface cracks and peelings as the Al-Si plate and the GA plate were experienced after hot forming. Therefore the substrate could be well protected.
Table 4: Test results of 720h neutral salt spray for test specimens
|
material |
Raw material weight(g) |
Weight after 720h of corrosion(g) |
weightlessness(g) |
Weight loss percentage |
Area /mm2 |
Area weightlessness (g/mm2) |
|
non-coated |
185.26 |
148.78 |
36.48 |
19.69% |
19006 |
1.9E-3 |
|
Al-Si |
149.69 |
147.15 |
2.54 |
1.69% |
18400 |
1.38E-4 |
|
GA |
241.73 |
237.57 |
4.16 |
1.72% |
10504 |
3.96E-4 |
|
electrogalvanized sheet after hot-forming |
235.85 |
235.74 |
0.11 |
0.4% |
19173 |
5.74E-6 |
 Figure 8 |
 Figure 9 |
 Figure 10 |
 Figure 11 |
Figure 8:hot-forming of uncoated steel for 720h salt spray test
Figure 9:hot-forming of Al-Si steel for 720h salt spray test
Figure 10:hot-forming of GA steel for 720h salt spray test
Figure 11:electrolytic galvanized parts of non-coated sheet after hot-forming for 720h salt spray test
3.2.2 Analysis of Scratch diffusion results of the coating samples
Results of the scratch diffusion results from 720h neutral salt spray experiment were shown in Table 5 and Figure 12 to 19.
From Figure 12 to 15, it could be seen that the width of corrosion products of Al-Si sheet of E-coating was the widest, up to 9.42mm, the next was the non-coated sheet, and the best was electrogalvanized parts of non-coated sheet after hot-forming.
From Fig. 16 to 19, the scratch width of Al-Si sheets after hot forming E-coating was 3.22mm, and the corrosion was developed along the depth direction and transverse direction. The reason was perhaps that the metal compounds formed after Al-Si hot-forming had a small amount of cathodic protection function. However, because of sacrificing anode protection effect, GA sheet and electro galvanized parts of non-coated sheet after hot-forming corroded the zinc layer first, which leads to the development of corrosion along the transverse direction.
Table5: corrosion results of scratches for 720h salt spray test
|
Coating |
Raw scratch width (mm) |
coating corrosion width for 720h (mm) |
Substrate corrosion width for 720h(mm) |
|
Non-coated |
1.20 |
8.51 |
1.54 |
|
Al-Si |
1.479 |
9.42 |
3.22 |
|
GA |
0.938 |
6.67 |
0 |
|
electrogalvanized sheet after hot-forming |
0.957 |
6.08 |
0 |

Figure 12
|

Figure 13
|

Figure 14
|

Figure 15
|

Figure 16
|

Figure 17
|

Figure 18
|

Figure 19
|
Figure 12:30hot-forming of non-coated steel for 720h salt spray test
Figure 13:31hot-forming of Al-Si steel for 720h salt spray test
Figure 14:32hot-forming of GA steel for 720h salt spray test
Figure 15:33hot-forming of electrolytic galvanized sheet after hot-forming for 720h salt spray test
Figure 16:Substrate corrosion width of hot-forming of uncoated steel for 720h salt spray test
Figure 17:Substrate corrosion width of hot-forming of Al-Si steel for 720h salt spray test
Figure 18:Substrate corrosion width of hot-forming of GA steel for 720h salt spray test
Figure 19:Substrate corrosion width of hot-forming of electro galvanized sheet after hot-forming for 720h salt spray test
3.2.3 Neutral salt spray experiment of B-pillar
Fig. 20 to 22show a non-coated B-pillar, an Al-Si coated laser tailor-welded B-pillar and a non-coated B-pillar galvanized parts, respectively. Fig. 23 to 25show the cut-out of the B-pillars referred to above, and Fig. 26 to 28show the 16-hour corrosion graphs of the neutral salt spray of the B-pillars mentioned above.
Comparing with Fig. 29 of the experiment mentioned above, it could be seen that when Al-Si coating was formed by flat die, a few cracks appeared on the surface of the coating, and only a few red rust appeared in 24h salt spray. During the hot forming process of B-pillar, the surface of B-pillar rubbed against the surface of the die, and the coating was severely damaged, as showed in Fig. 27. The corrosion resistance of Al-Si coated B-pillar was much worse. However, in the process of neutral salt spray test, galvanized B-pillar appeared no corrosion on the part substrate after hot forming.

Figure 20
|

Figure 21
|

Figure 22
|
 Figure 23 |
 Figure 24 |
 Figure 25 |
 Figure 26 |
 Figure 27 |
 Figure 28 |
 Figure 29 |
Figure 20:Non-coated B-pillar
Figure 21: Al-Si coating B-pillar
Figure 22: Galvanized B-pillar
Figure 23:Local area of non-coated B-pillar
Figure 24: Local area of Al-Si coating B-pillar
Figure 25:Local area of galvanized B-pillar
Figure 26: Local area of non-coated B-pillar for 2h salt spray test
Figure 27: Local area of Al-Si coating B-pillar for 16h salt spray test
Figure 28: Local area of galvanized B-pillar for 16h salt spray test
Figure 29 heating and air cooling of Al-Si steel (for 24h)
4 Conclusions
1. The E-coating layer had good corrosion resistance, but the protective effect on the round hole; trimming and edge of the parts were not good. When the E-coating layer was damaged, the hot-forming product without coating or with AL-Si coating had no corrosion resistance.
2. Al-Si coated sheet and GA sheet had excellent corrosion resistance, but after heating, the coating was cracked and the corrosion resistance was weakened. After hot-forming, the coating was easy to stick with the form die, and the coating thickness was reduced, and corrosion resistance was greatly reduced. Because of the thinner thickness and more severe crack, the corrosion resistance of the hot-forming parts of the GA plate was lower than that of the Al-Si plate.
3. Scratch experiments showed that the non-coated sheet corroded along the thickness direction of the substrate, while the corrosion direction of the Al-Si coated sheet develops along the thickness direction of the substrate and the lateral direction of the coating. The GA sheet by hot-forming and the electro galvanized parts of non-coated sheet after hot-forming, due to sacrificial anodic protection effect, mainly corrode in the lateral direction of the coating, and the substrate did not corrode. Therefore, in the vicinity of the non-coated layer of the coated sheet, the corrosion resistance of the GA sheet was higher than that of the Al-Si sheet due to the sacrificial anode protection, such as the outer scratched area, the vicinity of the cutting edge of the hole, the trimming edge of the part, the weld seam and the vicinity of the spot weld.
4. The coating of the electro galvanized parts of non-coated sheet after hot-forming was dense and uniform without damage (including the opening of the hole and the edge of the part), and had excellent cathodic protection effect, and its corrosion resistance was significantly better than Al-Si coated and GA coated hot-forming parts. Electro galvanized parts of non-coated sheet after hot-forming could protect the car body very well.
References
[1] K.Mori,D.Ito,Prevention of oxidation in hot-forming of quenchable steel by oxidation preventive oil,CIRP Annals-Manufacturing Technology,58(1),267(2009)
[2] J.Kondratiuk,P,Kuhn,MKoyer,M.Meurer,A ner coating solution for hot press forming,Galvatech 2011,Federica Bassani(Genova,AIM,2011)p.182
[3] J.Faderl,S.Kolnberger,M.Rosner,T.Kurz,Continuou galvanizing meets press-harding,Galvatech 2011,Federica Bassani(Genova,AIM)p.161
[4] D.W.Fan,H.S.Kim,J.Oh,K.Chin,B.C.de Cooman,Coating degradation in hot press forming,ISIJ International,4(50),2010
[5] Seinhoff K,Barbakedze N,Schupfer M.Press harding-from galvanized UHSS to body-in-white application[C]//8th International Conference on Zinc Alloy Coated Steel sheet.Genava:Galvatech,2011:12-15
[6] LAWERENCE C,Heeseung K,CHANGWOOK L,et al.Microstructure of liquid metal embrittlement cracks on Zn-coated 22MnB5 press-hardened steel[J].Scripta Materialia,2014,90-91;25-28.
[7] Bing.Li,Chun.zhang,Ming.wang.Hot Stamping of Boron Steel Plate with Al-Si Coating for Vehicle Bottom Longitudinal Beam.Metal heat treatment[J] .2017,42(6):39-42.
[8] Gui Zhongxiang,Liang Weikang,Zhang Yisheng.Formability of aluminum-silicon coated boron steel in hot-forming process[J].Transactions of Nonferrous Metals Society of China,2014,24(6):1750-1757.
[9] Xiaopan.qiu,Jie.zhang,Sheming.du.Effect of Stamping Temperature on Cracks in Galvanized 22MnB5 Steel Coating.Corrosion and Protection[J] .2018,39(4):302-306.
[10] J.Maki,M.Kurosaki,K.Kusumi,et al.Effect of Heating Condition and Hot Forming on Corrosion Resistance of Hot Satmped Aluminized Steels[A].Proceedings of 3rd International conference on hot sheet metal forming of high-performance steel.Kassel,Germany,2011,499-507.
[11] M.Fleischanderl,S.Kolnberger,J.Faderl,et al.Patent US20070256808,2007.



